We are thrilled to announce the launch of our latest innovative solution - the Clinical Trial Landscape! This AI-powered solution modernizes how medical professionals analyze global clinical trial data. By streamlining the complex process of exploring and synthesizing this information, our software enhances efficiency and effectiveness for medical science liaisons, medical affairs professionals, and clinical development teams.
Crafted with precision and designed to address the challenges of today's clinical trial landscape analysis, this innovative tool meets the crucial demand for inclusive, unbiased access to global clinical data and streamlines the process of uncovering and summarizing valuable insights.
Powerful AI, Combined With Comprehensive Data
Our Research Solutions Team is always looking to modernize conventional research methods. Remaining competitive in the dynamic realm of clinical research demands a shift away from laborious manual analysis. Our Clinical Trial Landscape solution accelerates the process, leveraging AI to streamline clinical trial landscaping.
Comprehensive Clinical Trial Registry Coverage
Our Clinical Trial Landscape solution ingests global coverage, ensuring all clinical data is accounted for. This is particularly beneficial for clinical researchers and scientists who rely on a comprehensive view of clinical trials for their analyses and reports. With our solution, users can access data from multiple registries in one place, allowing for more efficient and thorough research.
- Governmental Registries: These are maintained by government agencies, such as the U.S. National Institutes of Health (NIH) and the European Union Clinical Trials Register (EU CTR).
- Disease-Specific Registries: These focus on specific diseases or conditions, such as cancer or rare diseases.
- Institution-Based Registries: These are maintained by individual hospitals or research institutions.
Having access to comprehensive clinical trial registry coverage offers numerous benefits, such as more reliable data from multiple sources, a better understanding of trends and patterns in the trial landscape, and improved efficiency and productivity by accessing all the data needed in one place.
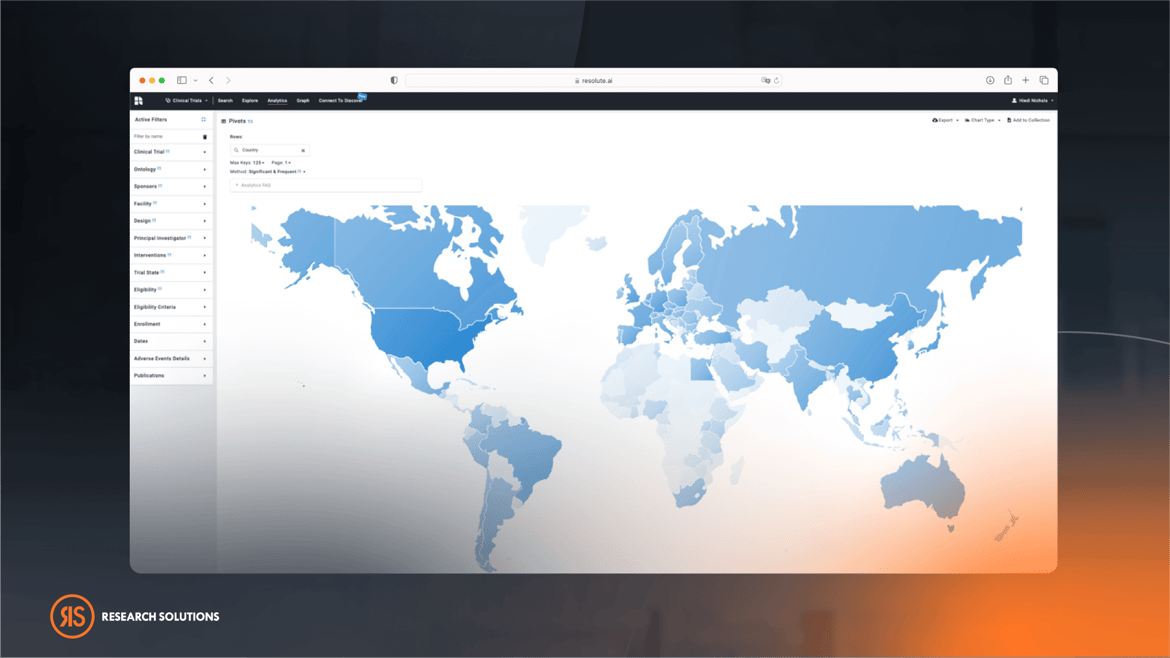
Unbiased Data With Agnostic Search
With the abundance of information available online, it can be challenging to sift through all the noise and find accurate and relevant data. This is where agnostic data searching becomes crucial. Agnostic data search refers to searching different sources of data without any inherent bias, ensuring all available data is considered, and users are presented with a complete and unbiased view of the information landscape. This includes lesser-known sources that might not be commonly used which opens up new avenues for research and provides unique and relevant information that might not be found on other platforms.
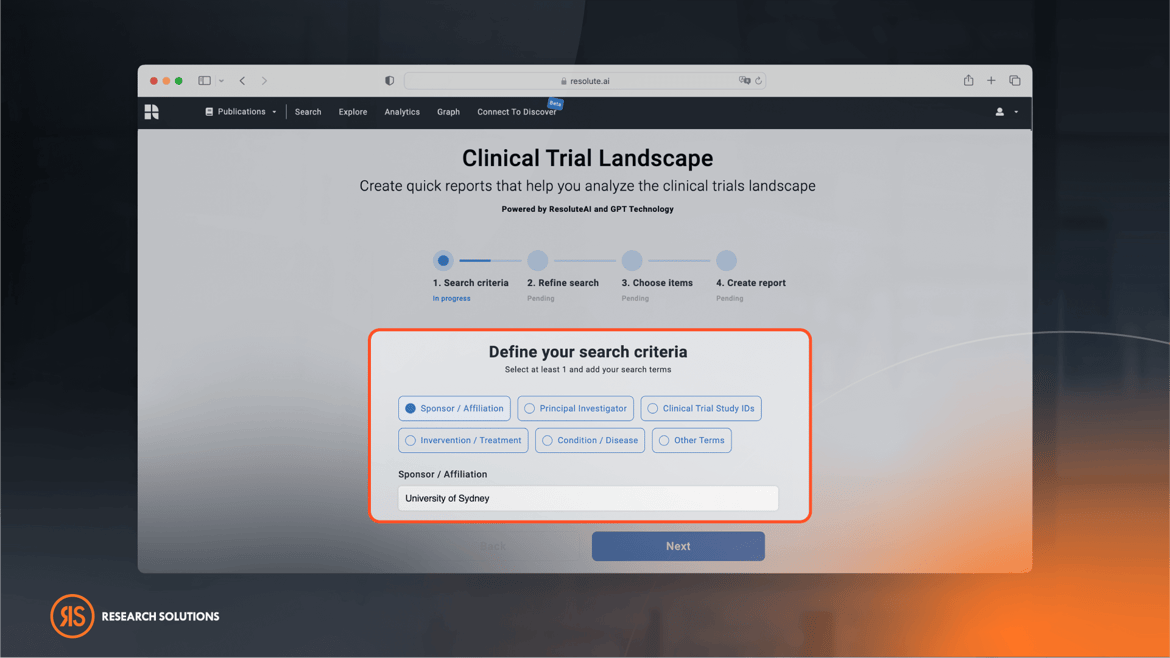
Advanced Filtering Options
With the ability to filter search results through various parameters, users can narrow down their research efficiently. Some of our sophisticated filtering options include:
- Start Date
- Sponsor Type
- Phase
- Status
- Intervention Model
- Intervention Name
- Eligibility Criteria
- Enrollment Count
- Adverse Events
- Associated Publications
This level of specification and control allows researchers to pinpoint specific clinical trial data that matches their research criteria closely.

Optimized Search Experience With Customizable Strategies
Users are empowered to develop and adjust their search strategies based on the outcomes of their initial searches. This adaptability benefits Feasibility Analysts and Clinical Scientists in refining their search queries and allows them to strategically optimize their search parameters to yield more relevant and precise results, ultimately enhancing the efficiency and effectiveness of their research processes.
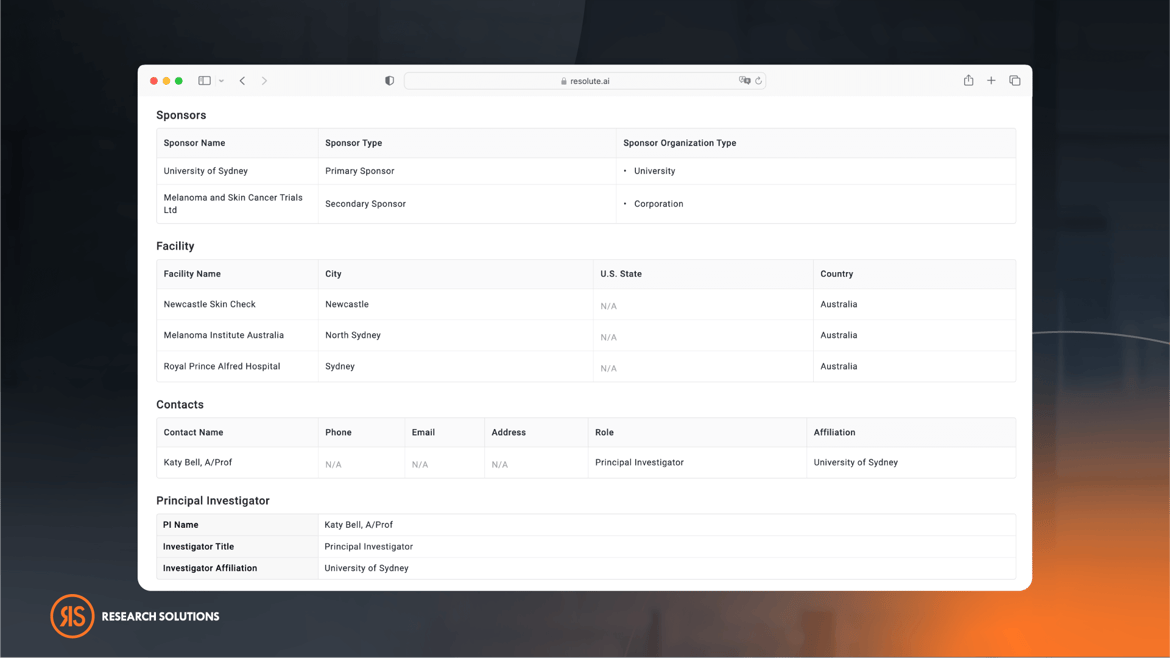
Understanding Clinical Trial Activity For Principal Investigators
The platform's sophisticated capability to assess the clinical trial experience of Principal Investigators (PIs) is invaluable for Medical Science Liaisons and Clinical Development professionals. By leveraging this feature, they can swiftly pinpoint and leverage critical expertise, streamlining their operations and fostering more effective collaborations within the medical research community.
Harness Data Efficiency In Clinical Trial Landscaping Today
There are two ways to begin experiencing the benefits of our Clinical Trial Landscape solution:
- Contact your Customer Success Manager to set up a 1:1 session to walk through these new software capabilities.
- Book a demo of our Clinical Trial Landscape solution with one of our experts to see the software in action and answer any questions you may have.
Live Product Launch Webinar: Streamlining Clinical Analysis To Accelerate Development
Discover how our innovative tool is transforming access to and analysis of clinical trial data by joining our live webinar. This session is not only the introduction of a new, intuitive tool, but also a leap towards the future of clinical research. No matter your role within clinical or medical disciplines, you'll find invaluable insights and modernized solutions to common challenges in your workflow. Secure your spot today!