Designed to modernize and streamline the systematic literature review workflow, Curedatis offers unparalleled advantages to medical device companies and other regulated industries. With fast article screening, simplified regulatory compliance, intelligent documentation, and comprehensive audit trails, our platform eliminates tedious manual tasks. This empowers users to search, discover, and manage clinical evidence effortlessly thanks to our AI engine.
With Curedatis, the time-consuming literature review process is no longer a barrier. Accelerate your clinical evaluation process and achieve better outcomes with ease.
Improve Your Processes With Effective Organization & Actionable Data
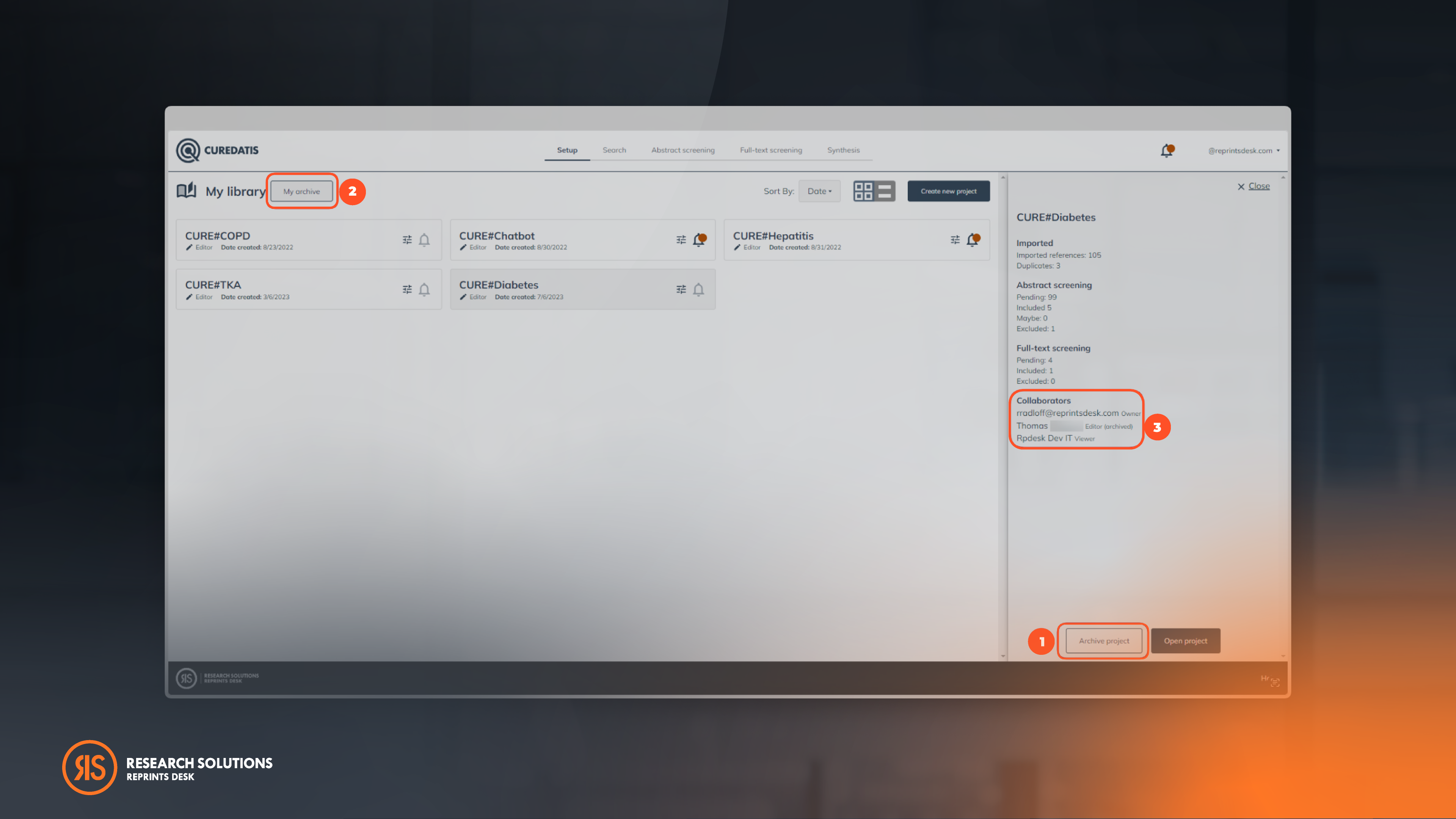
Archive Projects For A Clean & Organized Project Library
Users have the option to archive their projects in the designated "My Archive" section to enhance project management. Archiving a project will silence search alerts, providing a clutter-free workspace for active projects. Additionally, users can easily identify which collaborator has archived a specific project.
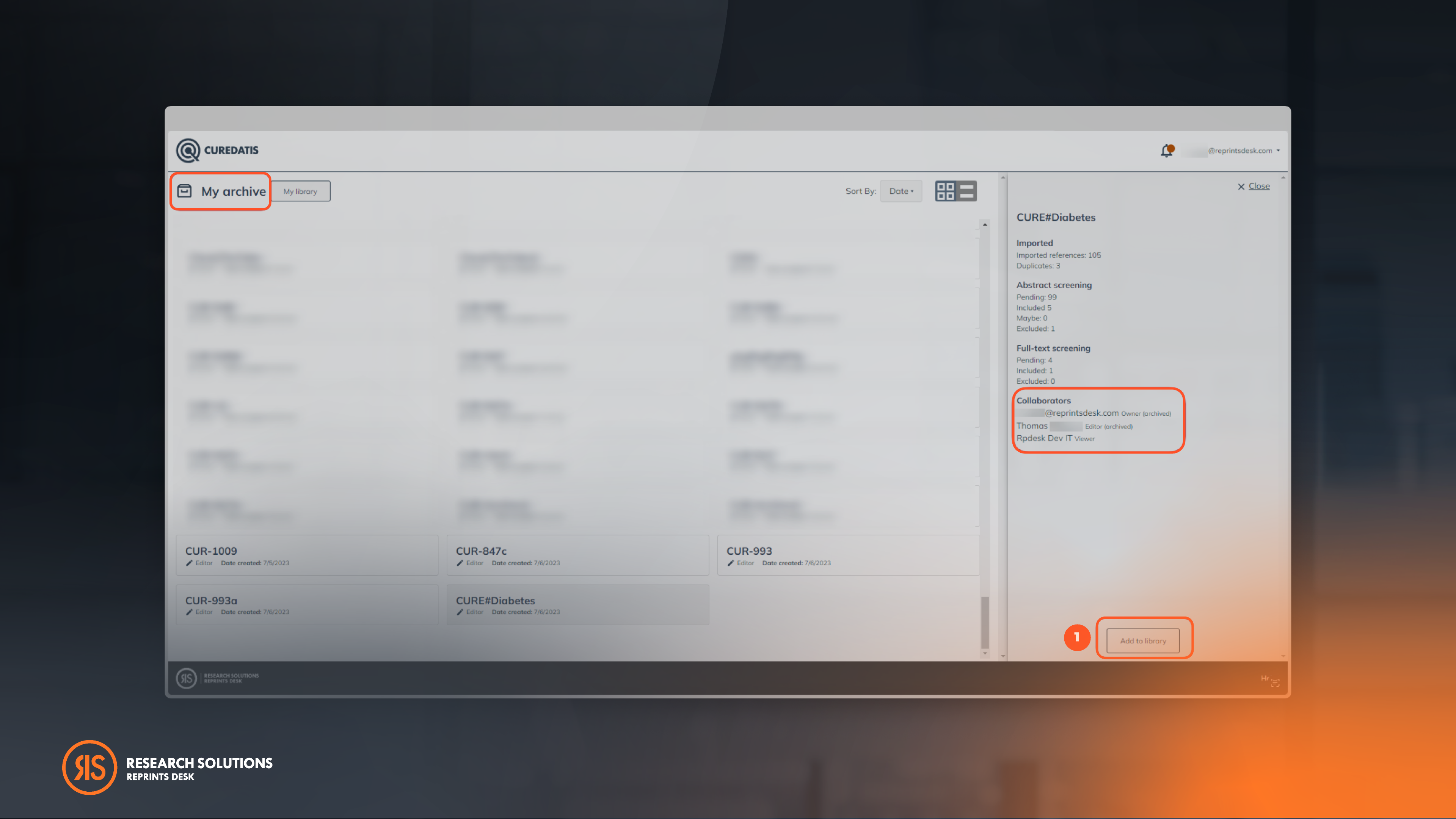
To activate projects, simply click "Add to library" under the "My Archive" section.
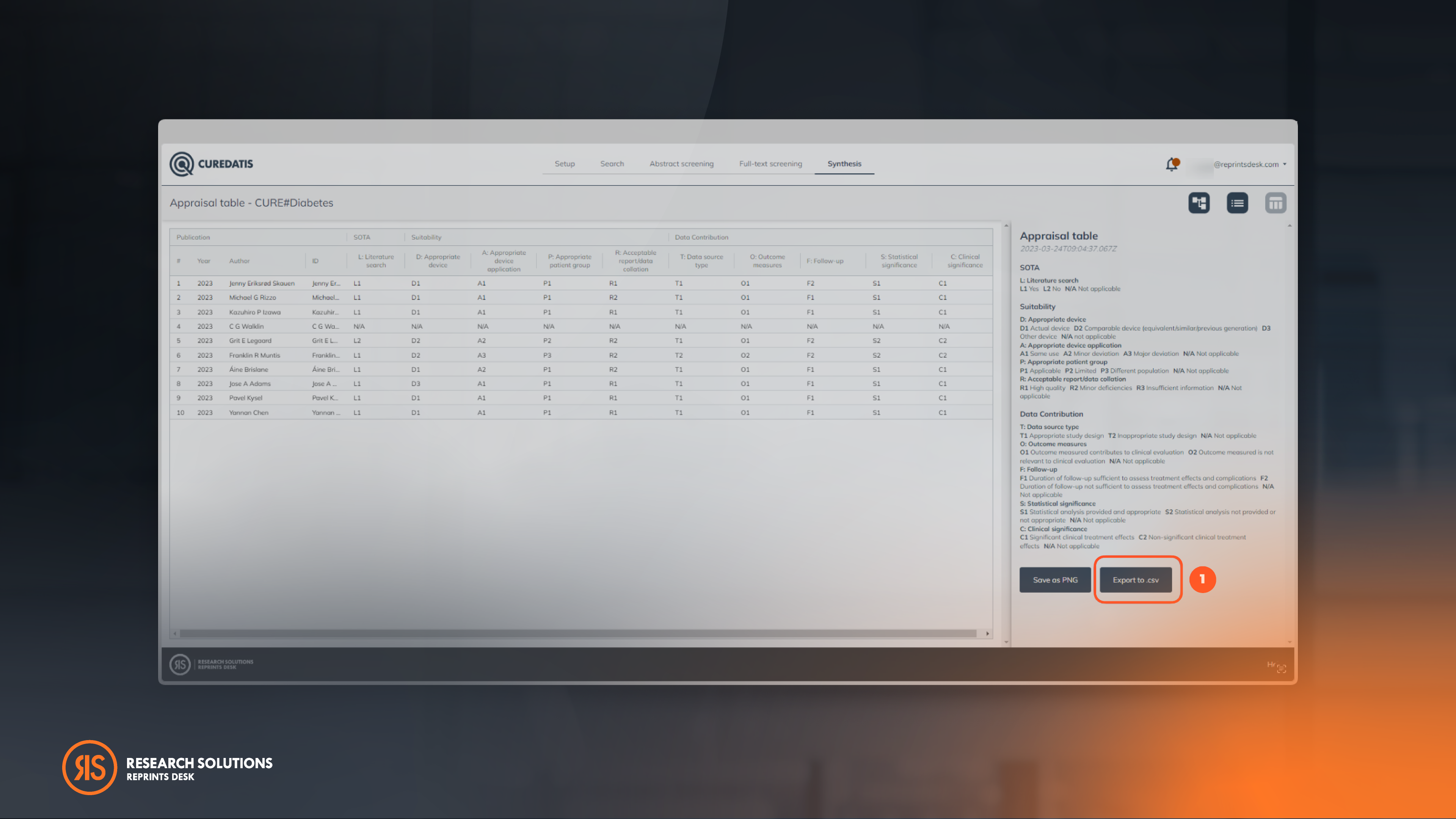
Access Actionable Data From Critical Appraisal
In addition to exporting the Appraisal Table as a PNG file, users now have the option to export the appraisal table in a CSV format for intelligent reporting. This file format can be easily utilized in popular spreadsheet tools such as Excel. This makes it easier to interpret data easier and allows for deeper analysis.
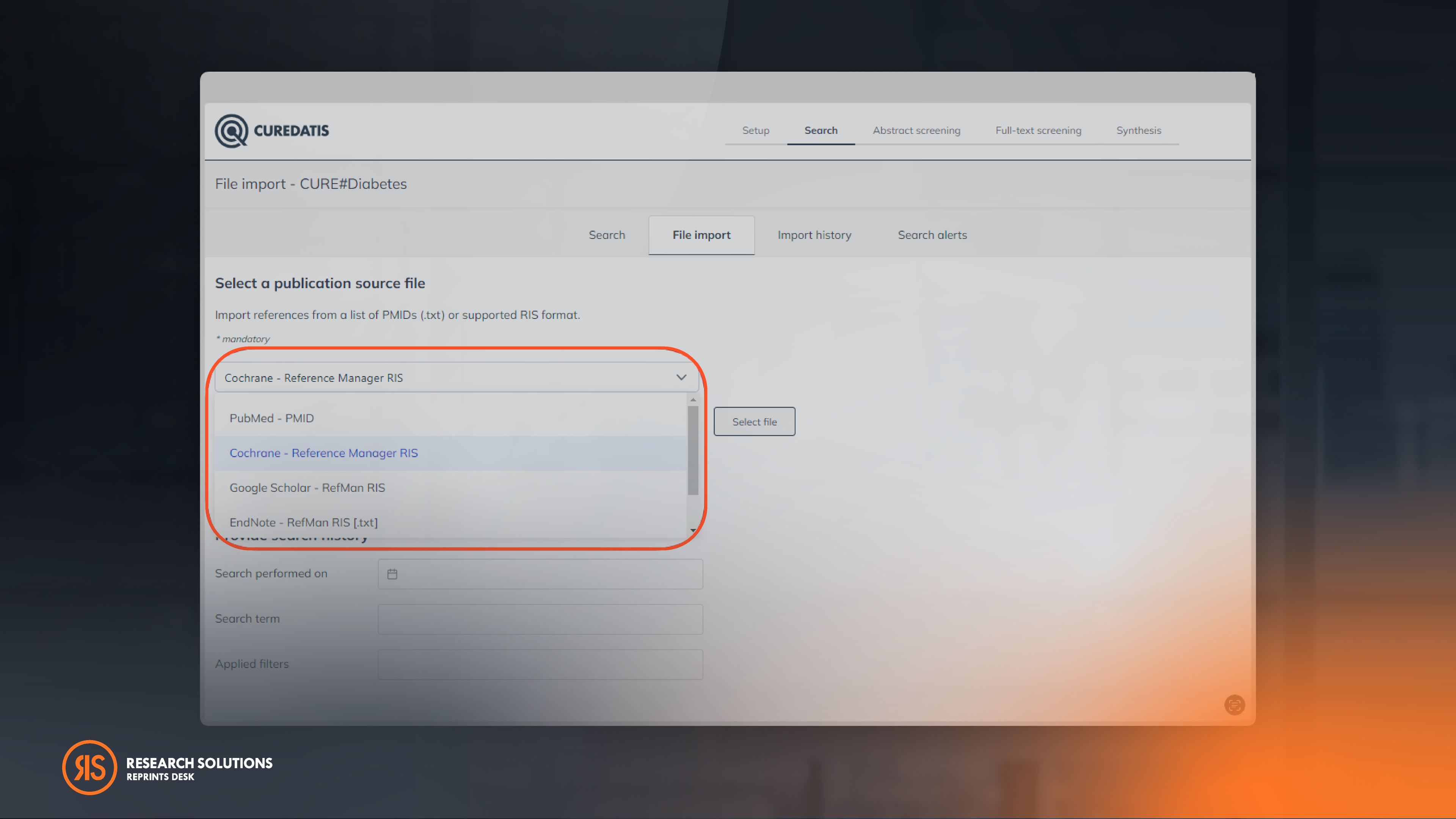
Unlock Broader Source Coverage With An Array Of Supported Import File Formats
Curedatis seamlessly imports various file formats, such as RIS from the Cochrane Library, EMBASE and Google Scholar. These search databases, among others, are used in many current research studies. This makes it easier for researchers to organize, store, and manage their citations in the way that works best for their specific needs.
Tip: To ensure top-notch import quality, use our Article Galaxy Browser Extension to generate a Google Scholar RIS file.
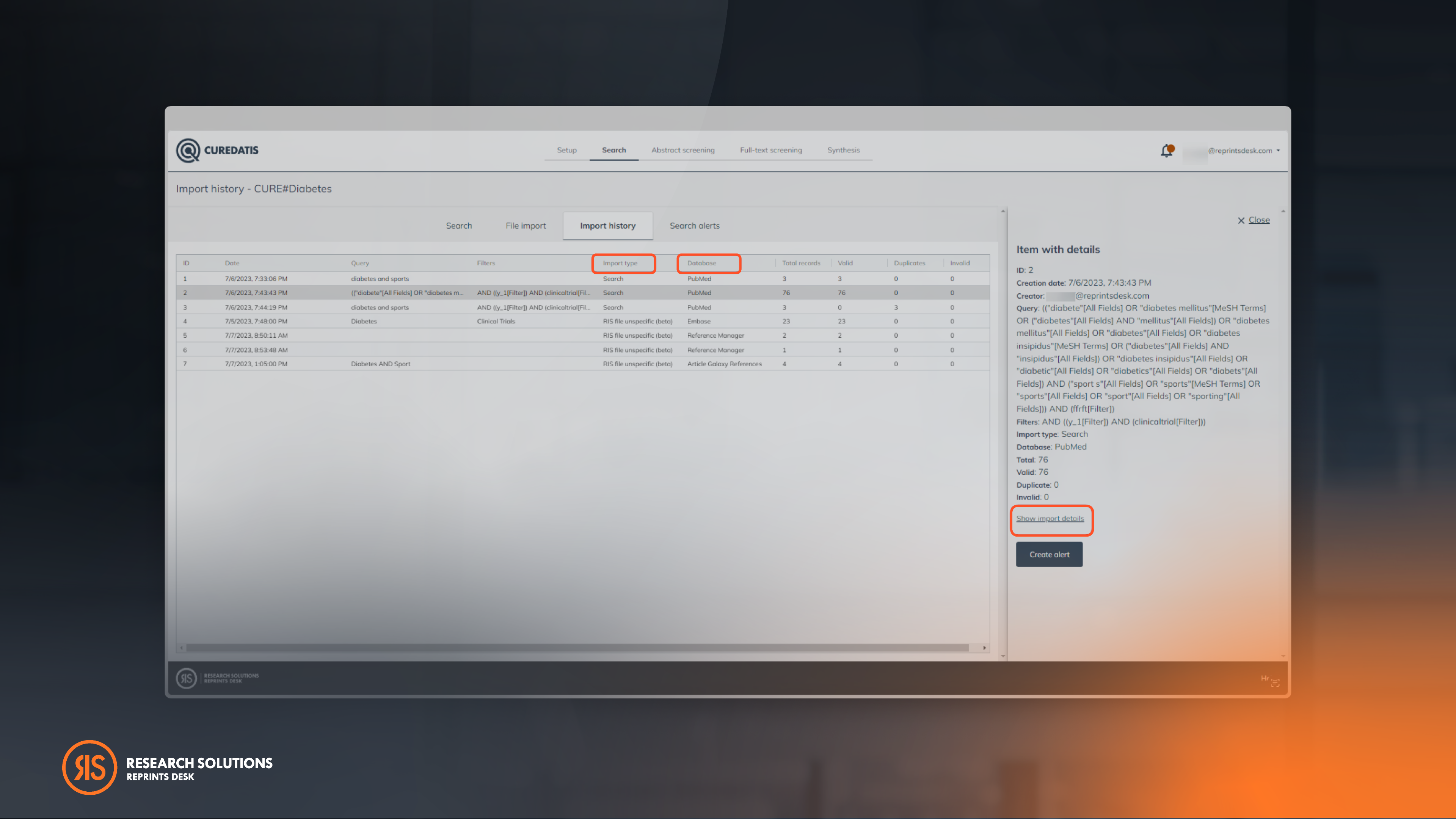
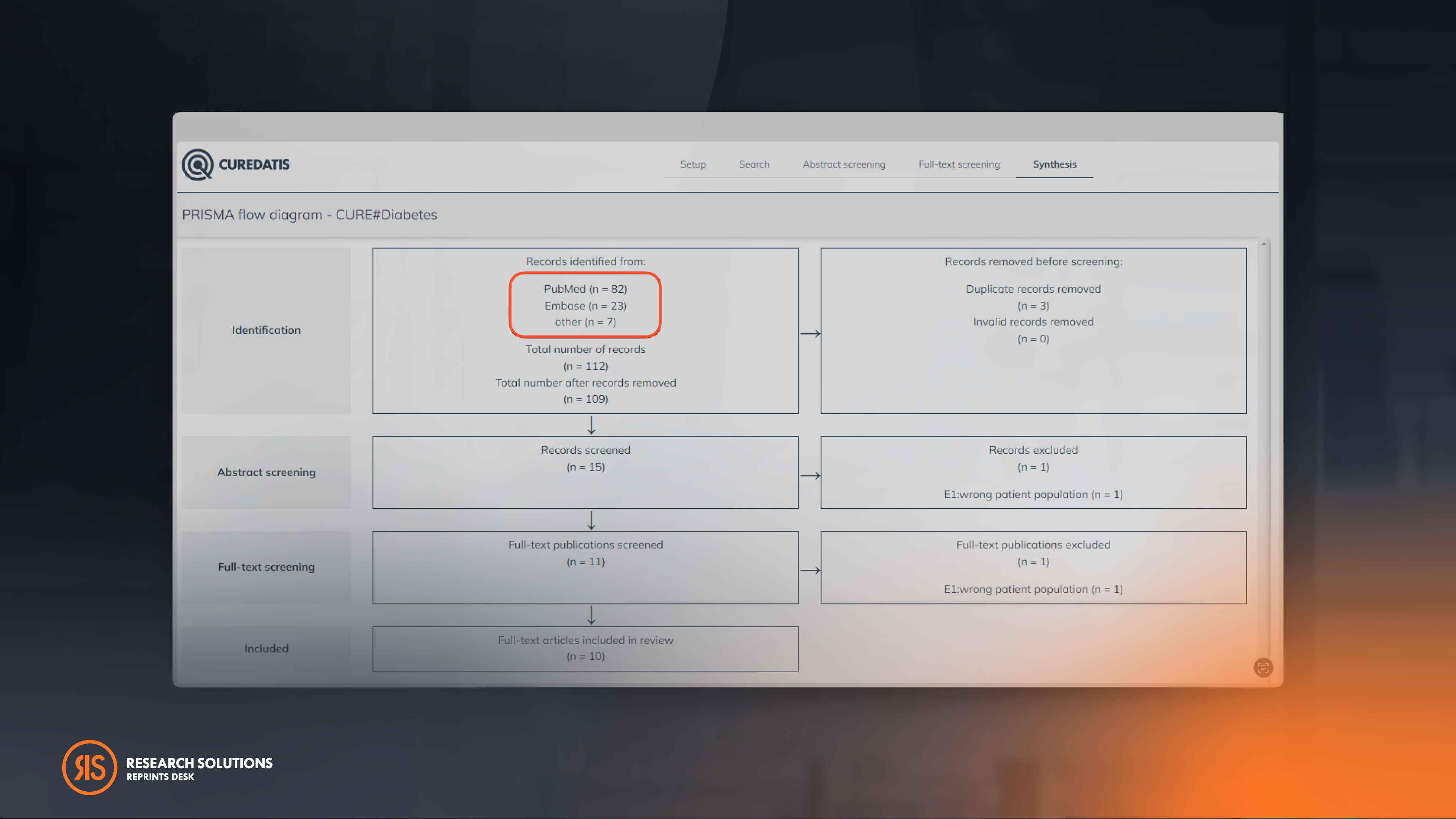
Improve Search Documentation Quality
Having streamlined search documentation allows researchers to quickly organize their collected data for easier analysis and interpretation. This is especially useful when conducting large-scale reviews.
Tip: View import details to see information about de-duplicated references. References are de-duplicated based on DOI or PMID.
Amplify Your Data Extraction With Our Customizable Forms
Our Customizable Data Extraction Form gives users the ability to annotate data in full-text and create personalized forms, delivering full control over projects. Simply navigate to the "Full-text screening" section, select the "Data Extraction" tab, and effortlessly add your desired "Category" and "Field." Maximize efficiency while maintaining the highest standards of accuracy and transparency, extracting the most reliable information from a wide range of sources.
Users can also now unlock advanced reporting methods by reviewing and exporting the extracted data in a convenient CSV format. To make this process even easier, a new "Extraction Icon" has been added under the "Synthesis" section.
.png?width=3840&height=2160&name=MicrosoftTeams-image%20(181).png)
.png?width=3840&height=2160&name=MicrosoftTeams-image%20(182).png)
Addressing What Matters Most To Your Organization
Become a Curedatis Development Partner
We value your input! Share your feature requests and feedback for Curedatis. We want to know how we can address the pain-points in literature reviews and clinical evaluations.
If you're interested in becoming a development partner, reach out to your account manager or contact our customer support team at customersupport@reprintsdesk.com.
Maximize the Benefits Of Our Newest Upgrades
We understand that every organization has distinct requirements when it comes to systematic literature reviews and clinical evaluations. Our comprehensive suite of tools and features can empower you to meet those needs and achieve success. Allow us to walk you through a personalized demo, where we will showcase the full potential of our powerful tools.
Book a demo today to have all your questions addressed and enhance your regulatory decision making with confidence and simplicity.